A colleague of mine from Colorado asked a very good question: Why on Earth do we use Gray tube tops for forensic blood analysis to determine Blood Alcohol Content for ETOH (drinking alcohol)?
The answer is evolution or really “re-purposing”.
It got me to thinking…
.jpg)
On the 24th day of November 1859, Charles Darwin published his seminal book entitled “On the Origin of Species“. It was where the well-accepted theory of biological evolution was first published. A little known fact is that the full title as it was originally published was: On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life.
I suggest that for Blood Alcohol testing for ETOH, the same pretty much happened. Here is why.

In order to understand why it is such a great and important question and to put the question into its proper perspective, you first have to know three (3) things:
(1) There is a growing trend across the United States is to move away from Evidentiary Breath Testing to determine Breath Alcohol Content (BrAC) using infrared spectrometry (i.e., breathalyzers) and towards true forensic blood alcohol testing using a Gas Chromatograph with usually a Flame Ionization Detector (to be distinguished from the forensically unacceptable practice of non-whole blood or Hospital Blood testing to determine Blood Alcohol Content); and,
(2) When we do true Blood Alcohol Content testing by drawing a person’s blood, the blood goes into a specimen tube; and,
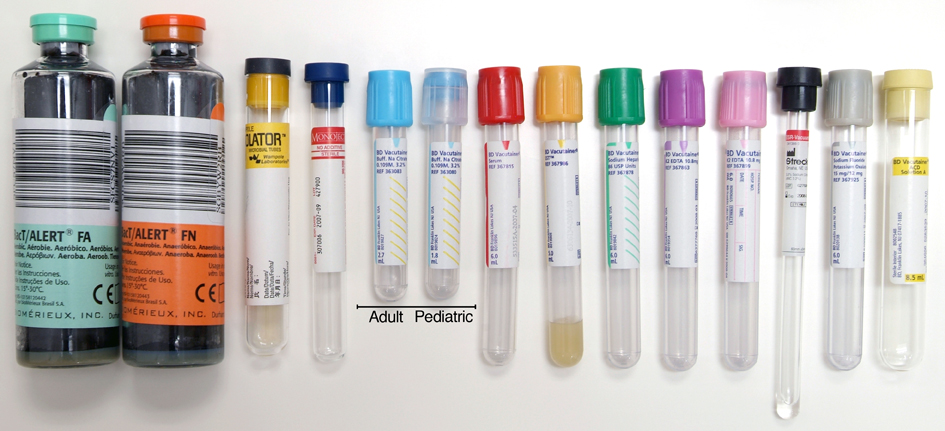
(3) There is more than one type of blood specimen tube (e.g., not all gray tube tops have the same thing in them)
As there are more than one type of tube, the great question why did we settle on the gray one?
But first some history….
What we have to remember is that chemical testing for ETOH content in both breath and blood and even urine is a relatively new and novel as far as the general field of science goes. New York in 1910 was the first jurisdiction in the United States of America to adopt laws against drunk driving, with California and others following. Early laws simply made illegal driving while intoxicated. It only required proof of a state of intoxication with no specific definition of what level of drunkenness qualified. The first per se law (where only the BAC was necessary to prove the offense) was established Norway in 1936. The first generally-accepted legal limit for blood alcohol concentration (BAC) was 0.15. Then by linking it to federal highway funding, the national limit was moved down to 0.10 and most recently to 0.08.
The study of alcohol as an academic exercise, however, can be traced to the late 1700s when J.J. Plenc proposed the chemical identification of poisons
In 1937, Indiana University’s Prof. Rolla N. Harger conducted the first-ever “short course” on chemical tests for intoxication. Shortly thereafter in 1938, Dr. Harger also introduced the Drunkometer, the first stable instrument for testing breath alcohol.
Professor Robert F. Borkenstein of Indiana University, in 1954, invented the Breathalyzer, the first practical instrument for testing breath alcohol. Whereas the The Drunkometer required re-calibration when it was moved from place to place, the Breathalyzer was more portable.
But I digress….
This blog is supposed to be about true Blood-based Blood Alcohol Content testing. Actually forensic blood testing for a Blood Alcohol Content is even “newer” than Breathalyzer technology. Especially considering Harold McNair did not really popularize Gas Chromatography in the United States until around 1967.
The technique of headspace-gas chromatography (HS-GC) was originally invented by Professor G. Machata of the University of Vienna in Austria. The proper instrumentation for the practical realization of the technique was developed by Perkin-Elmer’s German affiliate, using a unique pressure-balanced, time-based sampling of the headspace of the thermostatted sample vials. The first instrument, the Model F-40 was introduced in the fall of 1967; it was then followed by the Model F-42 in 1975 and the Model F-45 in 1978.
So, it is very, very new. It was some time much after that the forensic blood testing for ETOH content to determine Blood Alcohol Content really began.
This is the long way back around to the original question: Why gray tubes?
Therein the history of it all was the problem. We had an unpopular technique that has incrementally come on line so to speak. This presented a big problem.
That is because BD Vaccutainer and other blood tube specimen manufacturers did not develop tubes simply and specifically designed for ETOH determination.
Originally the NaF and K2C2O4 (sodium fluoride and potassium oxalate) tubes were developed for blood samples to test on the aspects of blood sugar tolerance, red blood cell electrophoresis, Alkali-Resistant HB Determination, and sugar dissolution. Due to the addition of the anti- glycolytic agent or inhibitor, these additives in the tube could maintain the blood specimen’s original characters for some time, and the metabolism of the RBC relative ceased. Glucose values in unpreserved blood samples decrease quickly after collection as glucose is metabolized by the blood cells. The additives contained in BD Vaccutainer Sodium Fluoride/Potassium Oxalate tubes may stop enzymatic activity at the glycolytic pathway if present in sufficient amounts, if properly inverted and properly refrigerated, transported and stored.
So if it is originally designed for these types of studies, how did it become used for ETOH determinations? Like much of the science in DUI, the glucose tube has been bastardized and re-purposed for ETOH testing through the years
It is not perfect and due to the ad hoc nature of this development and re-purposing there are a lot of unintended consequences that can develop that can call into question the accuracy, the precision, the reliability, the repeatability, the traceability and the truth of the reported Blood Alcohol Content result.
Eventually, I will post on these common forms of error.
-Justin J. McShane, Esquire, Pennsylvania DUI Attorney
I am the highest rated DUI Attorney in PA as Rated by Avvo.com
You can follow me on Twitter , Facebook or Linkedin.
yongsoo park says:
I was just asking myself the same question and stubbled upon your blog. interesting article and perspective. I do want to ask why grey top is still not widely used over Serum tubes (RED or SST gold). perhaps, its because of economical (cost of the tube) or the legal implication (the additives can have an adverse effect on the sample’s integrity).